Theoretical yield is 216.88 grams when reacting with 100.0 grams of Cl2 with excess CS2.
Step-by-step explanation:
Theoretical yield is the complete conversion of a limiting reagent in product.
In the equation given
3Cl2 + CS2 ⇒ CCl4
100 grams of Cl2 will give
number of moles =
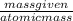
=
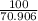
= 1.41 moles
3 moles of Cl2 gives 1 mole of CCl4
1.41 moles of Cl2 will give x moles of CCl4
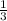 =
=
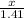
0.47 moles of CCl4 is formed
Theoretical yield is 0.47 x 153.82
= 216.88 grams.
100 gram of Cl2 is the limiting reagent in the reaction which produced 216.88 grams of CCl4.