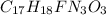Answer:
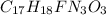
Step-by-step explanation:
In the figure attached, I have numbered the carbon atoms to permit to count them easily.
1. Carbon atoms
Each vertex without a letter represents a carbon atom:
- Count them: 17 (they are numbered on the figure attached)
2. Hydrogen atoms
For each carbon subract 1 for each bond and subtract 1 for each group attached to it:
- Carbon 1: 4 - 2 = 2
- Carbon 2: 4 - 2 = 2
- Carbon 3: 4 - 2 = 2
- Carbon 4: 4 - 2 = 2
- Carbon 5: 4 - 4 = 0
- Carbon 6: 4 - 4 = 0
- Carbon 7: 4 - 3 = 1
- Carbon 8: 4 - 4 = 0
- Carbon 9: 4 - 4 = 0
- Carbon 10: 4 - 3 = 1
- Carbon 11: 4 - 4 = 0
- Carbon 12: 4 - 4 = 0
- Carbon 13: 4 - 3 = 1
- Carbon 14: 4 - 3 = 1
- Carbon 15: 4 - 2 = 2
- Carbon 16: 4 - 2 = 2
- Carbon 17: 4 - 4 = 0
Total H atoms attached to C atoms:
- 2 + 2 + 2 + 2 + 1 + 1 + 1 + 1 + 2 + 2 = 16
Total H atoms in all: 16 + 2 = 18
3. Fluorine atoms
Count them:
4. Nitrogen atoms
Count them:
5. Oxygen atoms
Count them:
6. Molecular formula
Summary:
Molecular formula: