Answer:
Choice D) Platinum.
Step-by-step explanation:
The specific heat of a substance gives the amount of energy it takes to raise the temperature of
- unit mass (1 kilogram in this case) of the substance,
- by one unit (1 degree Kelvin in this case).
After energy of size
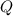 is added to a sample with specific heat
is added to a sample with specific heat
 and mass
and mass
 , the temperature change would be:
, the temperature change would be:
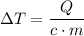 .
.
For each of these samples,
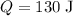 ,
,
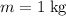 . Apply this equation to find the change in their temperatures.
. Apply this equation to find the change in their temperatures.
Aluminum
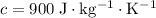 .
.
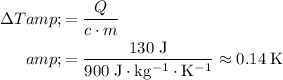 .
.
Copper
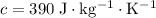 .
.
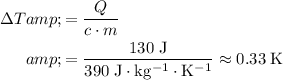 .
.
Brass
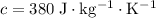 .
.
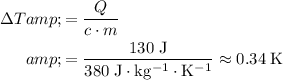 .
.
Silver
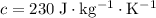 .
.
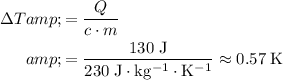 .
.
Platinum
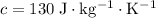 .
.
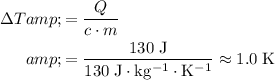 .
.
Hence, platinum would experience the greatest temperature increase.