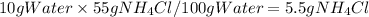Answer:
Please, see attached two figures:
- The first figure shows the solutility curves for several soluts in water, which is needed to answer the question.
- The second figure shows the reading of the solutiblity of NH₄Cl at a temperature of 60°C.
Step-by-step explanation:
The red arrow on the second attachement shows how you must go vertically from the temperature of 60ºC on the horizontal axis, up to intersecting curve for the solubility of NH₄Cl.
From there, you must move horizontally to the left (green arrow) to reach the vertical axis and read the solubility: the reading is about in the middle of the marks for 50 and 60 grams of solute per 100 grams of water: that is 55 grams of grams of solute per 100 grams of water.
Assuming density 1.0 g/mol for water, 10 mL of water is:
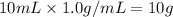
Thus, the solutibily is: