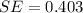Answer:
a. SE = 0.403 mol
Explanation:
The standard error of the mean, is the standard deviation of means of means.
It is calculated using,
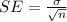
We have the standard deviation to be
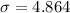
and the sample size is n=146.
We substitute the values to get:
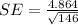
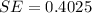
We approximate to three decimal places to get: