Answer:
- Option D. There will be a shift toward the reactants.
Step-by-step explanation:
The reaction is:
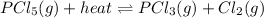
The application of LeChatelier's principle leads to consider the heat as a reactant or a product depending on if it is on the left side or the right side.
In this reaction, the heat is on the left side, thus it must be considered a reactant.
Decreasing the temperature is equivalent to remove or consume heat. Thus, the reaction must shif to the left to compensate that reduction of heat. That is the reverse reaction shall be favored.
In conclusion, there will be a shift toward the reactants.