178.87 grams of sulphur dioxide is formed according to the equation given.
Step-by-step explanation:
The balanced chemical reaction for the process is:
4 FeS2 + 11 O2 ⇒ 2Fe2O3(-) + 8 SO2
the reaction involving FeS and oxygen having balanced chemical equation as:
moles of oxygen given as 3.84
so when 11 moles of O2 reacts to form 8 moles SO2
3.84 moles of oxygen reacts to form x moles of SO2
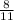 =
=
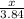
x =
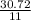
2.792 moles of sulphur dioxide will be formed
The mass can be calculated as number of moles x molar mass of 1 mole of SO2
= 2.792 x 64.066
= 178.87 grams of sulphur dioxide is formed.