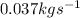Answer:
The mass flow rate of the fuel, in kg/s =
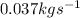
Step-by-step explanation:
The actual balanced equation for the combustion reaction can be written as :

At 95% theoretical air entering at 25° C and 1 atm, the equation of the reaction can be represented as :
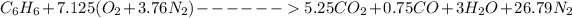
The Energy rate formula can be use to determine the mass flow rate of the fuel and which is given as:
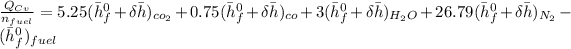
from ideal gas table at respective amount for each compound; we have:
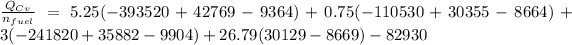
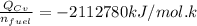
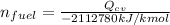
Given that;
The combustion products exit at 1000 K =
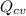
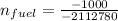

From the ideal gas tables M = 78.11 kg/kmol
∴
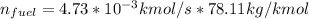
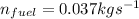
∴ The mass flow rate of the fuel, in kg/s =