Answer:
All the amounts of reactants and products are:
KBr
11.0g (given)
0.0924 mol
Cl₂
0.0462 mol
3.28g
KCl
0.0924 mol
6.89 g
Br₂
0.0462 mol
7.39 g
Step-by-step explanation:
1. Balanced chemical equation (given)
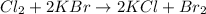
2. Mole ratios
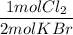
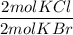
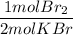
3. Molar masses
- Molar mass Cl₂: 70.906g/mol
- Molar mass KBr: 119.002 g/mol
- Molar mass KCl: 74.5513 g/mol
- Molar mass KBr: 159.808 g/mol
4. Convert 11 grams of potassium bromide to moles:
- #moles = mass in grams / molar mass
- #mol KBr = 11g / 119.002g/mol = 0.092435mol KBr
5. Use the mole ratios to find the amounts of Cl₂, KCl, and Br₂
a) Cl₂
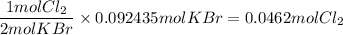

b) KCl
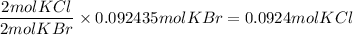
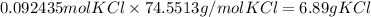
c) Br₂
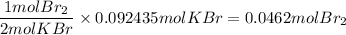
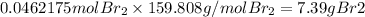
The final calculations are rounded to 3 sginificant figures.