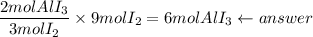Answer:
- 1. Iodine is the limiting reactant
- 2.
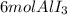
Step-by-step explanation:
1. Balanced chemcial equation:
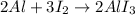
2. Theoretical mole ratio
It is the ratio of the coefficients of the reactants in the balanced chemical equation:
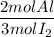
3. Actual ratio
It is ratio of the moles available to reat:
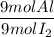
4. Comparison
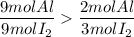
Then, there are more aluminum available than what is needed to react with the 9 moles of iodine, meaning that the aluminum is in excess and the iodine will react completely, being the latter the limiting reactant.
Conclusion: iodine is the limiting reactant.
5. How much aluminum iodide will be produced?
Use the theoretical mole ratio of aluminum iodide to iodide: