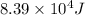Answer : The activation energy for the reaction is,
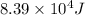
Explanation :
According to the Arrhenius equation,
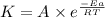
Taking logarithm on both side, we get:
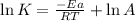
where,
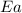 = activation energy for the reaction
= activation energy for the reaction
R = gas constant = 8.314 J/mole.K
T = temperature
K = rate constant
A = pre-exponential factor
The linear equation expression is:
y = mx + c
Plot of ln (K) versus (1/T) gives a straight line with a slope is equal to,
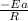
As we are given that:
Slope =

So,
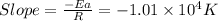
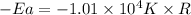
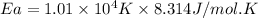
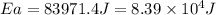
Thus, the activation energy for the reaction is,