Answer: The molar concentration of NaOH in the final diluted solution is 0.056 M
Step-by-step explanation:
To calculate the molarity of solution, we use the equation:
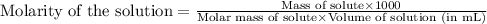
Given mass of NaOH = 4.00 g
Molar mass of NaOH = 40 g/mol
Volume of solution = 5.00 mL
Putting values in above equation, we get:
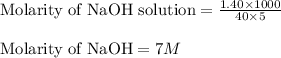
To calculate the molarity of the diluted solution, we use the equation:
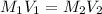
where,
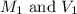 are the molarity and volume of the concentrated NaOH solution
are the molarity and volume of the concentrated NaOH solution
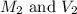 are the molarity and volume of diluted NaOH solution
are the molarity and volume of diluted NaOH solution
We are given:
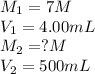
Putting values in above equation, we get:
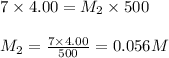
Hence, the molar concentration of NaOH in the final diluted solution is 0.056 M