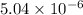Answer : The value of
 is
is
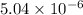
Explanation :
To calculate the concentration of
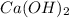 , we use the equation given by neutralization reaction:
, we use the equation given by neutralization reaction:
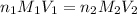
where,
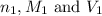 are the n-factor, molarity and volume of
are the n-factor, molarity and volume of
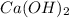
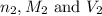 are the n-factor, molarity and volume of HCl.
are the n-factor, molarity and volume of HCl.
We are given:
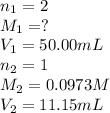
Putting values in above equation, we get:
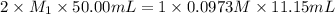
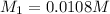
Now we have to calculate the
 for
for
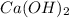
The solubility equilibrium reaction will be:
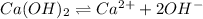
The expression for solubility constant for this reaction will be,
![K_(sp)=[Ca^(2+)][OH^(-)]^2](https://img.qammunity.org/2021/formulas/chemistry/high-school/t04syhg6kxetfccuucmeztzafqn7qvepuv.png)
Let the solubility be, x
x = 0.0108 M
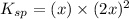
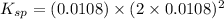
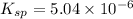
Therefore, the value of
 is
is