Answer:
1.25 cm/day
Step-by-step explanation:
An air thickness , (l) = 0.15 cm
Air Temperature =
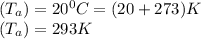
Mass Diffusion coefficient (D) =
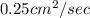
If the air pressure
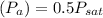
We are to determine how fast will the water
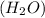 level drop in a day.
level drop in a day.
From the property of air at T = 20° C
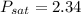 from saturated water properties.
from saturated water properties.
The mass flow of
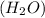 can be calculated as:
can be calculated as:
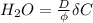
where:
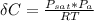
R(constant) = 8.314 kJ/mol.K
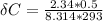
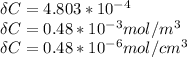
Since 1 mole = 18 cm ³ of water
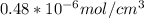 will be:
will be:
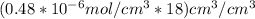
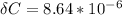
Again:
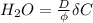
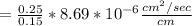
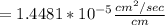
Converting the above value to cm/day: we have:
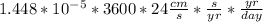
= 1.25 cm/day
∴ the rate at which the water level drop in a day = 1.25 cm/day