Answer: The mass of carbon in given amount of carvone is 47.52 grams
Step-by-step explanation:
To calculate the number of moles, we use the equation:
 .....(1)
.....(1)
Given mass of carvone = 55.4 g
Molar mass of carvone = 150.22 g/mol
Putting values in equation 1, we get:
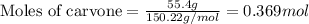
The chemical formula of carvone is
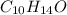
This contains 10 moles of carbon atom, 14 moles of hydrogen atom and 1 mole of oxygen atom
Now, calculating the mass of carbon in carvone from equation 1, we get:
Molar mass of carbon = 12 g/mol
Moles of carbon = (10 × 0.396) = 3.96 moles
Putting values in equation 1, we get:
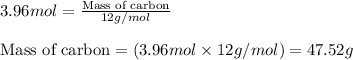
Hence, the mass of carbon in given amount of carvone is 47.52 grams