Answer:
The correct answer is 57.5 L
Step-by-step explanation:
According to the Charles's Law, as the temperature of a gas increases at constant pressure, its volume increases (the gas is expanded when it is heated). The mathematical expresion for two conditions (1 and 2) of a gas is the following:
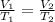
Where V₁ and V₂ are the volumes of the gas in two different conditions (1 and 2); T₁ and T₂ are the temperatures in Kelvin of the gas in the two conditions 1 and 2.
In this case, V₁= 46.0 L and T₁= 400 K. Then, we heat the gas until it reaches T₂=500 K. In order to calculate the new volume (V₂), we introduce the data in the mathematical expression:
V₂=
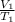 x T₂
x T₂
V₂= (46.0 L/ 400 K) x 500 K
V₂= 57.5 L
We corroborate our answer is coherent because 57.5 L > 46.0 L as we know that the volume increases with the increase of temperature (from 400 K to 500 K).