Answer:
Step-by-step explanation:
1. Barium hidroxide
Barium hydroxide is a strong base that ionizes as per the equation:
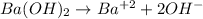
Thus, every mole of Ba(OH)₂ yields 2 moles of OH⁻.
2. Perchloric acid
Perchloric acid is a strong acid whose ionization may be represented by the following chemical equation:
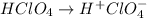
Thus, every mole of HClO₄ yields 1 mole of H⁺.
3. Calculate the number of moles of perchloric acid and H⁺:
- #moles of HClO₄ = Molarity × Volume in liters
- #moles HClO₄ = 0.167M × 25.4mL × 1L / 1,000m: = 0.0042418mol
The number of moles of H⁺ is equal to the number of moles of HClO₄
- #moles of H⁺ = 0.0042418 mol
4. Calculate the number of moles of Ba(OH)₂
When the solution is neutralized the number of moles of OH⁻ is equal to the number of moles of H⁺
- #moles of OH⁻ = 0.0042418
The number of moles of Ba(OH)₂ is half of the number of moles of OH⁻:
- #moles of Ba(OH)₂ = 0.0042418/2 = 0.0021209 mol Ba(OH)₂
5. Calculate the molarity of the Ba(OH)₂ solution:
- Molarity = number of moles / volume in liters
- Molarity = 0.0021209mol / 0.0128liters = 0.16569 ≈ 0.166M