Answer:
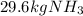
Step-by-step explanation:
Hello,
In this case, it is firstly necessary to know the limiting reactant via the shown below procedure:
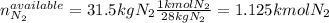
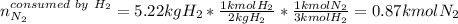
In such a way, the hydrogen is the limiting reagent as 5.22 kg of it do not consume all the available nitrogen, therefore, the theoretical yield of ammonia turns out as shown below:
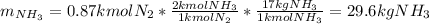
Best regards.