Answer:
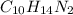
Step-by-step explanation:
Nicotine is a type of alkaloid that has two ring structures so-called as a bicyclic compound. In this compound, one ring is a pyridine cycle and the other is a pyrrolidine cycle. The molecular formula of this compound is
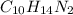 and this compound has three double bonds that are present in the pyridine cycle. It is a stimulant and addictive. It is used for different purposes such as treating tobacco disorder and some other.
and this compound has three double bonds that are present in the pyridine cycle. It is a stimulant and addictive. It is used for different purposes such as treating tobacco disorder and some other.