Answer:
2.27 M
Step-by-step explanation:
Given that :
volume = 250.0 mL = 0.250 L
Number of moles of
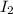 = 1.3 mol
= 1.3 mol
Number of moles of
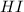 = 1.0 mole
= 1.0 mole
Initial concentration of
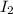 =
=
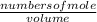
=
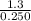
= 5.20 M
Initial concentration of
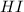 =
=
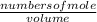
=
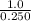
= 4.0 M
Equation of the reaction is represented as:
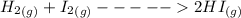
The I.C.E Table is as follows:
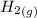 +
+
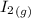 ----->
----->
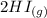
Initial(M) 0.0 5.20 4.0
Change +x +x - 2x
Equilibrium(M) x 5.20+x 4 - 2x
![K = ([HI]^2)/([H_2][I_2])](https://img.qammunity.org/2021/formulas/chemistry/college/v0tozcbyh2azsiq8eyje991g0zwre6ow18.png)
![K = ([4-2x]^2)/([x][5.20+x])](https://img.qammunity.org/2021/formulas/chemistry/college/sglh636iixzzlt52luf3hm5u9qlu5n6f0b.png) where K = 0.983
where K = 0.983
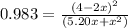
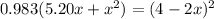
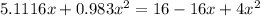
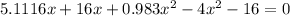
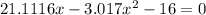
multiplying through by (-) and rearranging in the order of quadratic equation; we have:
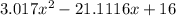
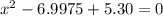
using the quadratic formula:
=
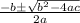
=
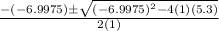
=
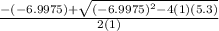 OR
OR
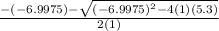
= 6.133 OR 0.864
since 6.133 is greater than K value then it is void, so we go by the lesser value which is 0.864
so x = 0.864 M
The equilibrium molarity of HI = (4.0- 2x)
= 4.0 - 2(0.864)
= 4.0 - 1.728
= 2.272 M
= 2.27 M to two decimal places