Answer:
The correct answer is 312.3 K
Step-by-step explanation:
In order to solve the problem, we use the modified Arrhenius equation which comprises two rate constants (k) and two temperatures (T):
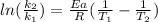
Where Ea is the activation energy (37.4 kJ/mol= 37400 J/mol), R is the gases constant (8.31 J/K.mol), k₁ and k₂ are the rate constant. We know k₁ (0.0160 s⁻¹) and from the problem, k₂ is twice k₁, so k₂= 2 x k₁= 2 x 0.0160 s⁻¹= 0.032 s⁻¹). Finally, T₁= 25ºC + 273 = 298 K. We introduce the data in the equation and calculate T₂:
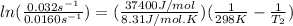
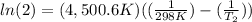
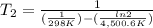
T₂= 312.33 K