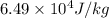Answer:
Latent heat of fusion of the substance is
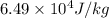
Step-by-step explanation:
Latent heat of fusion denotes amount of energy (heat) per unit mass required to melt a solid material at constant temperature and pressure i.e. at it's melting point
Here amount of heat required =
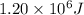
Mass of unknown substance being melted = 18.5 kg
So, latent heat of fusion of the substance = (required heat energy to melt)/(mass of the unknown substance) =
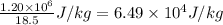
So, latent heat of fusion of the substance is