Answer:
There is 18.8% lithium by mass in second sample
Explanation:
Both the two samples are pure lithium carbonate. So % lithium by mass will be same in both samples. This can be proved mathematically as follows-
Let's assume weight of first sample is x g
So, 100 g of sample contains 18.8 g of lithium
Hence, x g of sample contains
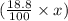 g of lithium
g of lithium
Now, the second sample has weight equal to 2x g
So, 2x g of sample contains
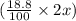 g of lithium
g of lithium
So, % lithium by mass in second sample =
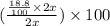 % = 18.8%
% = 18.8%