Answer:
576.21kJ
Step-by-step explanation:
#We know that:
The balance mass
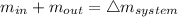
so,
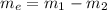
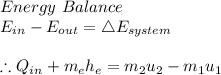
#Also, given the properties of water as;
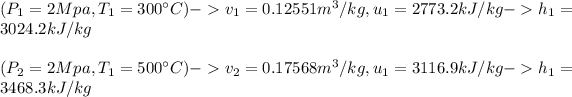
#We assume constant properties for the steam at average temperatures:
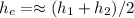
#Replace known values in the equation above;

#Using the mass and energy balance relations;
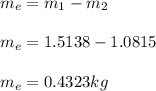
#We have
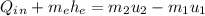 : we replace the known values in the equation as;
: we replace the known values in the equation as;
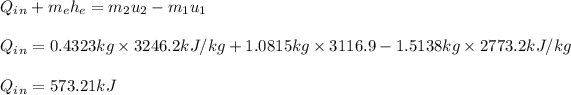
#Hence,the amount of heat transferred when the steam temperature reaches 500°C is 576.21kJ