The friction force f = 10000 N
The heat transfer Q = 1.7936 KJ
Step-by-step explanation:
Given data:
Surface area of Piston = 1
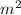
Volume of saturated water vapor = 100 K Pa
Steam volume = 0.05

Using the table of steam at 100 K pa
Steam density = 0.590 Kg/

Specific heat
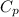 = 2.0267 KJ/Kg K
= 2.0267 KJ/Kg K
Mass of vapor = S × V
m = 0.590 × 0.05
m = 0.0295 Kg
Solution:
a) The friction force is calculated
Friction force = In the given situation, the force need to stuck the piston.
= pressure inside the cylinder × piston area
= 100 ×
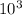 × 0.1
× 0.1
f = 10000 N
b) To calculate heat transfer.
Heat transfer = Heat needs drop temperatures 30°C.
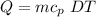
Q = 0.0295 × 2.0267 ×
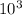 × 30
× 30
Q = 1.7936 KJ