Answer:
The resulting pressure of the air in the cylinder is 4 atmosphere.
Explanation:
Given : If a tire pump whose cylinder has a volume of 2.0 liters at 1 atmosphere of pressure compresses the air to a volume of 0.50 liters without releasing any of the air.
To find : What is the resulting pressure of the air in the cylinder?
Solution :
Let
 and
and
 represent the pressure and volume of the gas at an initial state .
represent the pressure and volume of the gas at an initial state .
 and
and
 represent the pressure and volume of the gas at an final state .
represent the pressure and volume of the gas at an final state .
According to Boyle's Law,

Substitute,
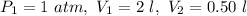
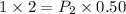
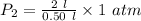
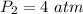
Therefore, the resulting pressure of the air in the cylinder is 4 atmosphere.