Work, W = 277.269kJ
Internal energy, Q = 277.269kJ
Step-by-step explanation:
Given-
Pressure, P1 = 2 bar
Temperature, T1 = 300K
Volume, V1 = 2m³
P2 = 1 bar
PV = constant
Let,
mass in kg be m
Work in kJ be W
Heat transfer in kJ be Q
R' = 8.314 kJ/kmolK
Mass of air, Mair = 28.97 kg/kmol
R = 0.289 kJ/kgK
We know,
PV = mRT
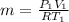
m = 5.65kg
To calculate V₂:
PV = constant = P₁V₁ = P₂V₂
P₁V₁ = P₂V₂
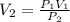
V₂ = 4m³
To calculate the work:
P₁V₁ = C
P₁ = C/ V₁
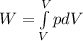
where limit is V₁ to V₂
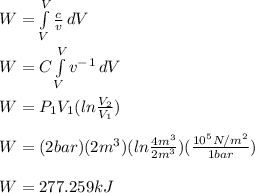
To calculate heat transfer:
Q - W = Δu
Q - W = m (u₂ - u₁)
Q = W + m (u₂ - u₁)
Q = W + m X cv X (T₂ - T₁)
Since, T₁ ≈ T₂
There is no change of internal energy.
W = Q
Q = 277.269kJ