Answer: Thus equilibrium partial pressure of CO is 1.306 atm
Step-by-step explanation:
 is the constant of a certain reaction at equilibrium.
is the constant of a certain reaction at equilibrium.
For the given chemical reaction:
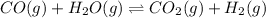
at t=0 2.50 2.50 1.00 1.00
at em (2.50-x) (2.50-x) (1.00+x) (1.00+x)
The expression of
 for above equation follows:
for above equation follows:
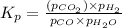
Putting values in above equation, we get:
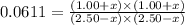
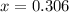
Thus equilibrium partial pressure of CO = (1.00+x) = (1.00+ 0.306) = 1.306 atm