Answer:
Acid :
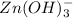
Conjugate base :
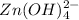
Base:

Step-by-step explanation:
According to the Lewis concept :
- An acid is defined as a substance that accepts electron pairs .
- Base is defined as a substance which donates electron pairs.
According to the Bronsted-Lowry conjugate acid-base theory:
- An acid is defined as a substance which looses donates protons and thus forming conjugate base .
- A base is defined as a substance which accepts protons and thus forming conjugate acid.
Given reaction :
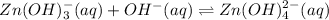
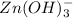 is acting as an acid because it is accepting hydroxide ion.And after accepting hydroxide ion it forms its conjugate base that is
is acting as an acid because it is accepting hydroxide ion.And after accepting hydroxide ion it forms its conjugate base that is
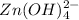
Since,
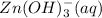 is acting as an acid
is acting as an acid
 will obviously act as base.
will obviously act as base.