Answer:
You will be able to use the sample for about 441 days.
Explanation:
The equation for the amount of polonium after t days is given by:
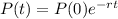
In which P(0) is the initial amount and r is the rate of decrease.
The half-life of polonium is 139 days
This means that
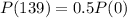 .
.
We apply this to the equation, and find r.
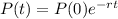
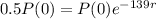
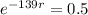
Applying ln to both sides of the equality:
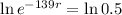
So
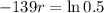
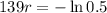
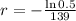
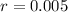
So
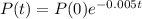
Your sample will not be useful to you after 89% of the radioactive nuclei present on the day the sample arrives has disintegrated. For about how many days after the sample arrives will you be able to use the polonium?
It will be useful until t in which
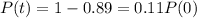 . So
. So
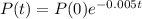
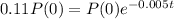
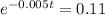
Applying ln to both sides
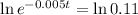
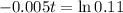
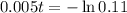
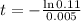
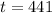
You will be able to use the sample for about 441 days.