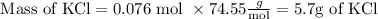5.7 g of KCl is produced.
Step-by-step explanation:
First we have to write the balanced equation as,
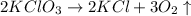
Then we have to find the moles of potassium chlorate as,
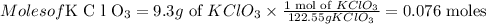
Then we have to find the moles of KCl as,
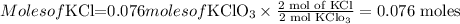
From the moles of KCl, we have to find the mass using its molar mass by multiplying them as,