The answers are as follows,
1. 4.71 L,
2. 3.29 L,
3. 1634.6 torr
Step-by-step explanation:
All the above problems can be sorted out using the Boyle's law represents the relationship between the volume and pressure.
It can be expressed as follows,
P1V1 = P2V2
Rearranging the above expression, we get,
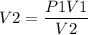
1) The first case can be solved as,
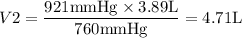
2) The second case can be solved as,
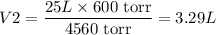
3) The third case can be solved as,
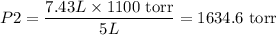
Thus the answers for all the three cases are found as,
1. 4.71 L,
2. 3.29 L,
3. 1634.6 torr respectively