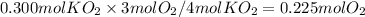Answer:
Step-by-step explanation:
1. Balanced chemcical reaction (given):
- 4KO₂ + 2CO₂ → 2K₂CO₃ + 3O₂
2. Mole ratio
Use the coefficients from the balanced chemical equation to form the mole ratio of O₂ to KO₂:
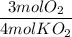
3. How many moles of O₂ are produced when 0.300 mol of KO₂ reacts in this fashion?
Mutiply 0.300 mol of KO₂ by the mole ratio: