Answer:
There are 0.00390 moles of Barium in 2.35 × 10²¹ atoms of Ba.
General Formulas and Concepts:
Stoichiometry
- Mole ratio
- Avogadro's Number: 6.023 × 10²³ atoms/moles/molecules/formula units/etc.
- Dimensional analysis
Step-by-step explanation:
Step 1: Define
Identify.
2.35 × 10²¹ atoms Ba
Step 2: Convert
- [Dimensional Analysis] Set up:
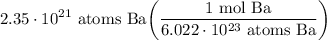
- [Dimensional Analysis] Evaluate:
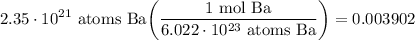
- [Sig Figs] Round:
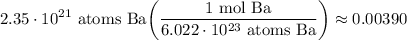
Topic: AP Chemistry
Unit: Stoichiometry