Answer:
The answer to the question is;
The average rate of the reaction over the 11 seconds time interval is
1.455×10⁻³ mol/s.
Step-by-step explanation:
To solve the question, we note that
Quantity of O₂ produced = 1.6×10⁻² mol
Reaction time = 11 seconds
Volume of reaction = 0.5 L
The rate of a chemical reaction is given by
Average rate of reaction =
![(d[O_2])/(dt)](https://img.qammunity.org/2021/formulas/chemistry/high-school/8q5p7yxq6cy6ml6sefir92enxf16xx8baz.png)
Average rate of production of O₂ k =
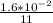 = 1.455×10⁻³ mol/second.
= 1.455×10⁻³ mol/second.