Answer : All flask contains the same number of molecules.
Explanation :
As we know that at STP,
Pressure of flask = 1 atm
Temperature of flask = 273 K
Volume of flask = 1 L
The given molecules are,
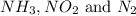
Every molecule contains equal number of molecules that is, 1.
That means all the value of variables are same. Thus, all flask contains the same number of molecules.
Hence, all flask contains the same number of molecules.