Step-by-step explanation:
It is known that atomic number of carbon is 6 and its electronic configuration is
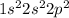 . This means that in its neutral state it contains 2 electrons in its s-orbital and 2 electrons in its p-orbital.
. This means that in its neutral state it contains 2 electrons in its s-orbital and 2 electrons in its p-orbital.
After excitation there will be one electron present in its s-orbital and three electrons present in p-orbital.
Therefore, after the hybridization there will be in total 2 sp hybrid orbitals, 2 p-orbitals and zero s-orbital.