Answer:
The empirical formula of the compound is =
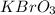
The name of the compound is potassium bromate.
Step-by-step explanation:
Mass of potassium = 4.628 g
Moles of potassium =
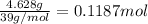
Mass of bromine = 9.457 g
Moles of bromine =
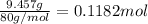
Mass of oxygen = 5.681 g
Moles of oxygen =
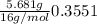
For empirical formula of the compound, divide the least number of moles from all the moles of elements present in the compound:
Potassium :
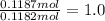
Bromine;
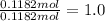
Oxygen ;
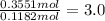
The empirical formula of the compound is =
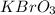
The name of the compound is potassium bromate.