Answer: The temperature change that occurred is 7.62°C
Step-by-step explanation:
The equation used to calculate heat absorbed follows:
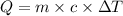
where,
Q = heat absorbed = 18.9 J
m = mass of oxygen gas = 2.6 g
c = specific heat of oxygen gas = 0.954 J/g°C
 = change in temperature = ?
= change in temperature = ?
Putting values in above equation, we get:
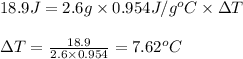
Hence, the temperature change that occurred is 7.62°C