Answer:
Alpha Boundary contains 87.33% of A and 12.66% of B
Beta Boundary contains 20.67% of A and 79.33% of B
Step-by-step explanation:
For the two alloys with composition as given in the question the values of the Weightage is given as
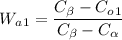
Here
Wa1 os given as 0.59 as value of Alpha Phase for 1st Alloy
Co1 is given as 60% for the first Alloy so the equation becomes
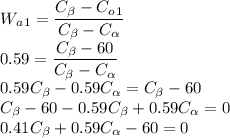
Similarly for the 2nd alloy
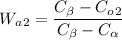
Here
Wa2 os given as 0.14 as value of Alpha Phase for 1st Alloy
Co1 is given as 30% for the first Alloy so the equation becomes
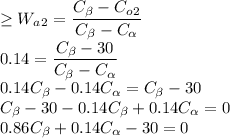
Solving the two equations as
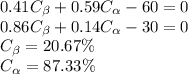
So the boundaries are as
Alpha Boundary contains 87.33% of A and 12.66% of B
Beta Boundary contains 20.67% of A and 79.33% of B