Number of moles of solute in 16.55 mL of 0.1475 M M2Cr3O7 is 0.00244 moles.
Step-by-step explanation:
The properties and behavior of many solutions depend not only on the nature of the solute and solvent but also on the concentration of the solute in the solution. Molarity (M) is the concentration of a solution expressed as the number of moles of solute per liter of solution:
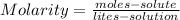 ,
,
Here ,
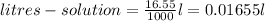 .
.
⇒
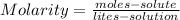
⇒
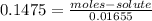
⇒
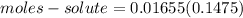
⇒
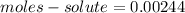
Therefore, Number of moles of solute in 16.55 mL of 0.1475 M M2Cr3O7 is 0.00244 moles.