Answer:
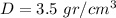
Explanation:
Density
The density of an object of mass m and volume V is given by
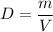
It can be expressed in common units like
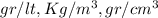 or any other combination of proper mass [M] by volume [V] units.
or any other combination of proper mass [M] by volume [V] units.
The data provided in the question is
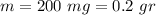
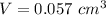
Thus, the measured density is
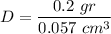
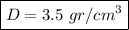
We have expressed the result with 1 decimal place because the mass was measured to the nearest hundred milligrams (or one-tenth grams). Any further decimal is senseless because that precision comes from calculations, not from measurements.