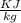Answer:
A. The work done during the process is W = 0
B. The value of heat transfer during the process Q = 442.83
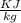
Step-by-step explanation:
Given Data
Initial pressure
 = 100 k pa
= 100 k pa
Initial temperature
 = 25 degree Celsius = 298 Kelvin
= 25 degree Celsius = 298 Kelvin
Final pressure
 = 300 k pa
= 300 k pa
Vessel is rigid so change in volume of the gas is zero. so that initial volume is equal to final volume.
⇒
 =
=
 ------------- (1)
------------- (1)
Since volume of the gas is constant so pressure of the gas is directly proportional to the temperature of the gas.
⇒ P ∝ T
⇒
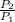 =
=
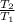
⇒ Put all the values in the above formula we get the final temperature
⇒
 =
=
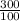 × 298
× 298
⇒
 = 894 Kelvin
= 894 Kelvin
(A). Work done during the process is given by W = P × (
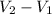 )
)
From equation (1),
 =
=
 so work done W = P × 0 = 0
so work done W = P × 0 = 0
⇒ W = 0
Therefore the work done during the process is zero.
Heat transfer during the process is given by the formula Q = m
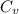 (
(
 -
-
 )
)
Where m = mass of the gas = 1 kg
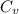 = specific heat at constant volume of nitrogen = 0.743
= specific heat at constant volume of nitrogen = 0.743
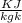
Thus the heat transfer Q = 1 × 0.743 × ( 894- 298 )
⇒ Q = 442.83
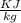
Therefore the value of heat transfer during the process Q = 442.83