Answer:
The dimerization of butadiene to 4-vinylcyclohexene folows second order kinetics and its rate law will be given by :
![R=k[C_4H_6]^2](https://img.qammunity.org/2021/formulas/chemistry/college/1i8gr2bnfhgp9lji9pgn00dn0heohjx340.png)
Step-by-step explanation:
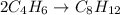
The rate of the reaction ;
![R=k[C_4H_6]^x](https://img.qammunity.org/2021/formulas/chemistry/college/6tci2kedlsxqmk2ch02z12b56o2ljrwzry.png)
As given in the question , that graph of time verses
![(1)/([C_4H_6])](https://img.qammunity.org/2021/formulas/chemistry/college/qxh7ayyxlijwa3usyscnvy8np9gtz82308.png) was linear but plots of
was linear but plots of
![[C_4H_6]](https://img.qammunity.org/2021/formulas/chemistry/college/fm84ltscls83bgvfnrsom0cw2k91hwit94.png) or
or
![\ln[C_4H_6]](https://img.qammunity.org/2021/formulas/chemistry/college/xusinmo79agkx6syqymufhh2tpbqp6oziz.png) was curved.
was curved.
Generally:
Graph of time verses
![[concentration]](https://img.qammunity.org/2021/formulas/chemistry/college/35i4nqrtkuvz22p4lrj8u3pndbmi33dv0q.png) for zero order reaction is linear with negative slope.
for zero order reaction is linear with negative slope.
Graph of time verses
![\ln [concentration]](https://img.qammunity.org/2021/formulas/chemistry/college/o1cyxvml1w75115dbp8ase9zm2508j0gnw.png) for secon order reaction is linear with negative slope.
for secon order reaction is linear with negative slope.
Graph of time verses
![(1)/([concentration])](https://img.qammunity.org/2021/formulas/chemistry/college/h1qsbn0tcibb22h2izpm9inszt4l9ewsvx.png) for secon order reaction is linear with positive slope.
for secon order reaction is linear with positive slope.
So, the dimerization of butadiene to 4-vinylcyclohexene folows second order kinetics and its rate law will be given by :
![R=k[C_4H_6]^2](https://img.qammunity.org/2021/formulas/chemistry/college/1i8gr2bnfhgp9lji9pgn00dn0heohjx340.png)