Answer:
A= 203 KJ
B= 54 Kg
Step-by-step explanation:
The initial specific volumes and internal energies are obtained from A-12 for a given pressure and state. The enthalpy of the refrigerant in the supply line is determined using the saturated liquid approximation for the given temperature with data from A-11. The mass that has entered the tank is:
Δm = m₂ – m₁
= V(1/α₂ – 1/α₁)
= 0.05 (1/0.0008935 – 1/ 0.025645)Kg
= 54Kg
The heat transfer is obtained from the energy balance:
ΔU=
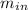
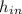 +
+
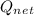
m₂u₂ – m₁u₂ =
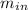
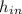 +
+
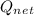
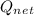 = m₂u₂ – m₁u₁ –
= m₂u₂ – m₁u₁ –
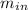
= V/α₂u₂ - V/α₁u₁ –
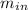
=(0.05/0.0008935 . 116.72 – 0.05/0.025645 . 246.82 – 54.108.28) Kj
= 203 KJ