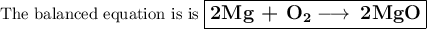Answer:
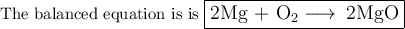
Step-by-step explanation:
Mg + O₂ ⟶ MgO
1. Balance O.
We have 2 atoms of O on the left and 1 O on the right.
Put a 1 in front of O₂ and a 2 in front of MgO.
Mg + 1O₂ ⟶ 2MgO
2. Balance Mg:
We have fixed 2 Mg on the right. We need 2 Mg on the 2 Mg on the left. Put a 2 in front of Mg.
2Mg + 1O₂ ⟶ 2MgO
Every formula now has a coefficient. The equation should be balanced.
3. Check that atoms balance:
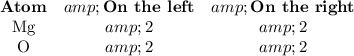
The equation is now balanced.