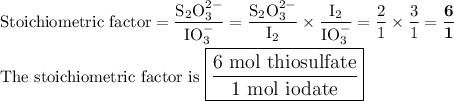Answer:
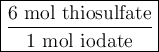
Step-by-step explanation:
The I₂ is the common substance in the two equations.
(1) IO₃⁻ + 5I⁻ + 6H⁺ ⟶ 3I₂ + 3H₂O
{2) I₂ + 2S₂O₃²⁻ ⟶ 2I⁻ + S₄O₆²⁻
From Equation (1), the molar ratio of iodate to iodine is
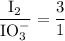
From Equation (2), the molar ratio of iodine to thiosulfate is
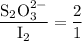
Combining the two ratios, we get