Answer:
7.92 .g . mL^-1
Step-by-step explanation:
Data provided in the question
Water liters = 45.5 mL
Pace of iron = 28.5 gram
Rise in the water in the cylinder = 49.1 mark
So by considering the above information
As we know that
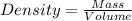
where,
Mass = 28.5 gram
Volume = 49.1 - 45.5
= 3.6 mL
So, the density is
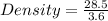
= 7.92 .g . mL^-1
Basically we use the density formula by dividing the mass with the volume