Answer: The concentration of potassium permanganate solution is 0.015 mM
Step-by-step explanation:
To calculate the molarity of solution, we use the equation:
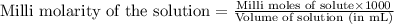
We are given:
Moles of solute =
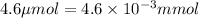 (Conversion factor:
(Conversion factor:
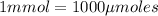
Volume of solution = 300 mL
Putting values in above equation, we get:

Hence, the concentration of potassium permanganate solution is 0.015 mM