The pH of the solution is 2.54.
Step-by-step explanation:
pH is the measure of acidity of the solution and Ka is the dissociation constant. Dissociation constant is the measure of concentration of hydrogen ion donated to the solution.
The solution of C₆H₂O₆ will get dissociated as C₆HO₆ and H+ ions. So the molar concentration of 0.1 M is present at the initial stage. Lets consider that the concentration of hydrogen ion released as x and the same amount of the base ion will also be released.
So the dissociation constant Kₐ can be written as the ratio of concentration of products to the concentration of reactants. As the concentration of reactants is given as 0.1 M and the concentration of products is considered as x for both hydrogen and base ion. Then the
![K_(a)=([H^(+)][HB] )/([reactant])](https://img.qammunity.org/2021/formulas/chemistry/middle-school/qjftgqybly2regfrfpccqa4v5xx8sjmkg4.png)
[HB] is the concentration of base.
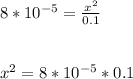

Then
![pH = - log [x] = - log [ 0.283 * 10^(-2)]\\ \\pH = 2 + 0.548 = 2.54](https://img.qammunity.org/2021/formulas/chemistry/middle-school/yuv7929c6w5dnu6i8s9dgz3wyl5yiwwtme.png)
So the pH of the solution is 2.54.